AACE/ACE Diabetes Algorithm for Glycemic Control, HbA1c 6.5% to 7.5%
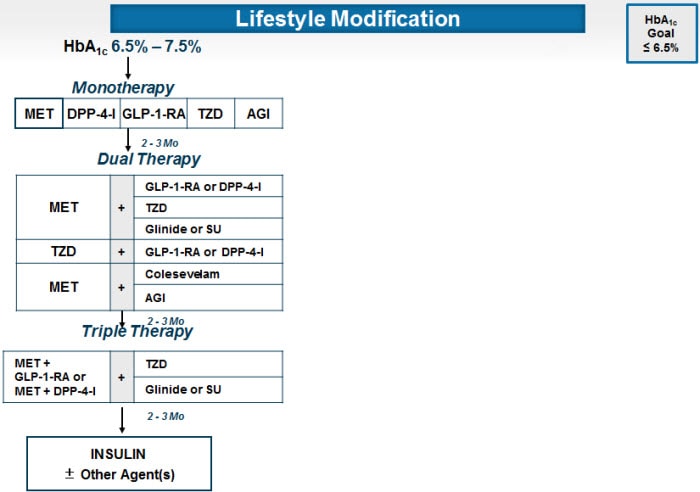
The AACE/ACE algorithm considers the incretin-based forms of therapy -- dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists -- as possible first-line agents to be used as monotherapy. Both are efficacious in terms of reduction of HbA1c. Both have extremely good safety profiles in terms of low risk for adverse events and hypoglycemia. These agents are highly recommended for monotherapy when metformin is contraindicated. GLP-1 receptor agonists require injections (twice daily for exenatide, once daily for liraglutide, or once weekly for exenatide QW).
Basal insulin may be associated with hypoglycemia in patients with HbA1c values in this range (<7.5%). Insulin is almost always associated with weight gain and occasionally fluid retention.
Sulfonylureas such as glimepiride may result in hypoglycemia and weight gain. The potential for hypoglycemia is of particular concern in this HbA1c range (≤7.5%). There is also the possibility that sulfonylureas could result in acceleration of the loss of beta-cell function compared with other agents, as was suggested by results from the UKPDS. In contrast to most other algorithms, including the recent 2012 American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) position statement, the AACE/ACE algorithm does not include sulfonylureas as an option for monotherapy, in view of the risk of hypoglycemia, limited durability of effectiveness, and the likelihood of weight gain.
Intensification of treatment is indicated to try to achieve a target HbA1c of 6.5% in patient with recent-onset diabetes and no diabetic complications. Because the major factor contributing to the elevation of HbA1c in this range of HbA1c is postprandial excursion, agents such as DPP-4 inhibitors and GLP-1 receptor agonists that improve postprandial hyperglycemia are preferred (target < 140 mg/dL).
Incretin-based agents are usually associated with minimal risk of hypoglycemia. The DPP-4 inhibitors are weight-neutral; the GLP-1 receptor agonists are generally associated with significant weight loss and occasionally (especially in the morbidly obese patient) with major weight loss. The DPP-4 inhibitors and the GLP-1 receptor agonists have the advantage that they stimulate beta cells to secrete insulin in a glucose dependent manner. This means that as the plasma glucose falls into the normoglycemic range, the effect of the incretin-based medication is reduced or turns off. Thus, as glucose decreases from an elevated level (eg, 150 mg/dL) to a normal or near-normal value (eg, 90 mg/dL), the enhancement of insulin secretion by the incretin-based therapy is reduced. Similarly, inhibition of glucagon secretion by the pancreatic alpha cells turns off as glucose levels fall to normal or low levels, so that glucagon counter-regulation comes back into play when needed.
The extended-release and branded forms of metformin may have better gastrointestinal tolerability than generic metformin.
Intensification of therapy with an insulin secretogogue, such as glipizide or basal insulin, increases the risk of hypoglycemia and weight gain. These agents may also hasten the decline of beta-cell function.
The ADA recommends the use of MNT as an important tool for helping patients with type 2 diabetes achieve therapeutic goals, although it can be a complex intervention for patients. Therefore, patients require educational programs, such as those provided by a CDEs; these clinicians can help patients become active participants in their disease management.
Several studies have evaluated the implementation of MNT and demonstrated that their use results in reductions in A1c and low-density lipoprotein (LDL) cholesterol. As little as a 5% reduction in weight decreases insulin resistance, improves glycemia and lipemia, and reduces blood pressure.
Studies have also shown that diet alone, diet and exercise, and meal replacement can result in sustained weight loss of 4.8% to 8% in patients with type 2 diabetes. The lack of a strict adherence to interventions is a major barrier to achieving recommended goals. Even a 10% decrease in total adherence to therapy with metformin has been shown to produce an increase of 0.14% in A1c.
The DAWN study showed that only 16.2% of patients with type 2 diabetes report being compliant with all the instructions provided by their healthcare providers.
Glycemic Recommendations for Non-pregnant Adults With Diabetes.
| A1c | < 7.0% |
| Preprandial capillary plasma glucose | 70-130 mg/dL (3.9-7.2 mmol/L) |
| Peak postprandial capillary plasma glucose | < 180 mg/dL (<10.0 mmol/L) |
General Comments
| |
Specific methods for improving adherence include:
- Verifying patient recall and comprehension of the treatment plan
- Clarifying potential treatment benefits
- Simplifying regimens
- Electronic monitoring
- Minimizing costs
- Discussing adverse events
- Minimizing diabetes-related depression or emotional distress
The ADA and European Association for the Study of Diabetes (EASD) consensus guidelines recommend the addition of insulin in patients whose A1c remains further from the target value of greater than 8.5% or who have symptoms related to hyperglycemia, following the use of oral therapy and lifestyle modification. Therapy should be individualized and insulin can be initiated earlier in the course of disease, as appropriate to lower A1c. The role and rationale for injection therapy was discussed with TC in an earlier visit and barriers to injection were addressed. Other injection therapies, such as the GLP-1 receptor agonists, may help promote weight loss.
Counseling Techniques for Challenging Barriers to Insulin Therapy
| Patient Statement | Clinician Response | Technique |
|---|---|---|
| "High blood sugar doesn't make me feel tired." | "I would like to know more details about how you feel. For instance, what is it like for you when you get up in the morning?" | Acceptance Questioning Clarification |
| "I just don't want to use insulin." | "I respect your feelings about that. Can you tell me why it bothers you so much?" Or "I wonder if you would do a short-term experiment to see what insulin does for you. Then you can make an informed decision about insulin." | Acceptance Questioning Negotiation Empowerment |
| "Well I tried it. Now what?" | "Congratulations. I'm impressed that you were willing to try something new. What you did --taking insulin -- has normalized your blood sugars. I bet you can apply this success to your other efforts-diet and exercise." | Esteem-building Cognitive framing Empowerment |
| "Having to use insulin must mean I'm getting worse" | "I can understand how you could conclude that. What's happening to you is actually quite normal for people who have had diabetes as long as you have. The purpose of insulin is prevention, not progression." | Acceptance Verification Normalization Positive reframing |
| "Can't I just try a stronger pill?" | "I know it would be easier if you could. Unfortunately, we don't have any stronger pills. I want to reassure you that insulin is what your body needs at this time. Can you try thinking of insulin as the most appropriate medication at this time to give your body?" | Validation Acceptance Support and information Positive reframing |
DPP-4 Inhibitors
One approach to incretin-based therapy involves blocking the degradation of endogenous incretins through the inhibition of dipeptidyl peptidase-4 (DPP-4). The DPP-4 inhibitors include sitagliptin, saxagliptin, and linagliptin, which are currently approved for the treatment of T2DM, as well as the investigational agents vildagliptin and alogliptin. DPP-4 inhibitors can be taken orally without regard to meals.
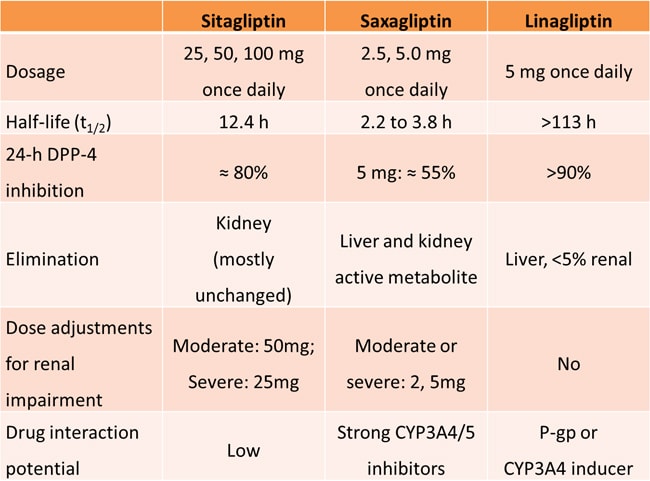
The efficacy and safety of DPP-4 inhibitors have been well studied in patients with T2DM. Saxagliptin added to metformin reduces HbA1c (P < .0001), fasting plasma glucose (P < .0001), and postprandial glucose (P < .0001) after 24 weeks compared with metformin alone. Linagliptin significantly reduces HbA1c by -0.5% to -0.7% when given as single-agent therapy or when added to metformin; thiazolidinedione (TZD); sulfonylurea; and metformin and sulfonylurea. In a head-to-head trial, sitagliptin added to metformin showed similar glucose-lowering effects after 52 weeks of treatment compared with glipizide added to metformin, but with a more favorable effect on body weight (1.5 kg weight loss vs 1.1 kg weight gain; P < .001) and a reduced risk of hypoglycemia (4.9% vs 32.0%).
GLP-1 Receptor Agonists (RAs)
GLP-1 RAs are indicated for the treatment of T2DM as an adjunct to lifestyle modifications including diet and exercise. Currently available agents include twice-daily exenatide, once-weekly exenatide, and once-daily liraglutide. GLP-1 analogs improve glycemic control in patients with T2DM by enhancing glucose-dependent insulin secretion and reducing postprandial glucagon secretion. The GLP-1 RAs also slow gastric emptying, resulting in increased satiety, reduced food intake, and weight loss. However, decreased gastric emptying may also lead to nausea, vomiting, and diarrhea for some patients.
Table 4. Properties of GLP-1 Agonists
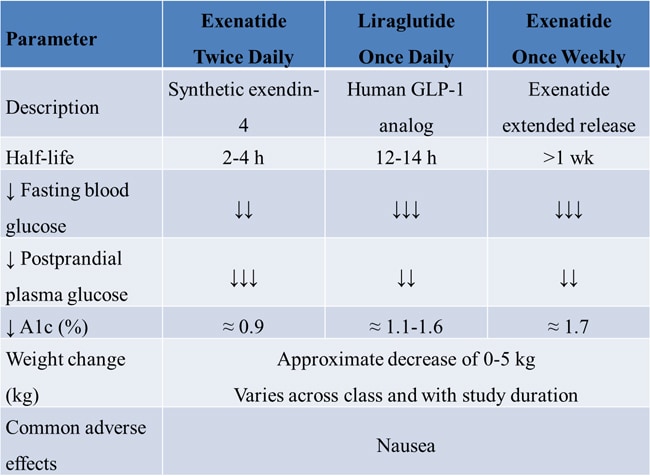
Liraglutide is a long-acting subcutaneous GLP-1 analog that is administered once daily. The LEAD-6 (Liraglutide Effect and Action in Diabetes) trial compared liraglutide 1.8 mg once daily and exenatide 10 µg twice daily in patients with poorly controlled T2DM despite treatment with metformin and/or a sulfonylurea. Add-on treatment with liraglutide provided a greater reduction in HbA1c compared with add-on exenatide (1.1% vs -0.8%; P < .05) and a greater reduction in fasting plasma glucose (-29.0 mg/dL vs -10.8 mg/dL; P < .05). Moreover, more patients in the liraglutide group than in the exenatide group reached the treatment goal of HbA1c < 7.0% (54.0% vs 43.0%; P < .05). Both agents were well-tolerated, and liraglutide and exenatide promoted similar levels of weight loss (-3.24 kg vs -2.87 kg).[33]
Newer formulations with reduced administration frequency may help improve patient adherence to antidiabetes therapy. The DURATION-1 (Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly) trial evaluated long-term treatment with different formulations of exenatide in patients with T2DM. Patients were randomly assigned to treatment with exenatide 2 mg once weekly or exenatide 10 μg twice daily for 30 weeks, followed by 1.5 years of exenatide 2 mg once weekly for all patients. Compared with twice-daily exenatide, treatment with once-weekly exenatide provided a greater reduction in mean HbA1c (-1.5% vs -1.9%). The DURATION-5 trial compared exenatide twice daily with exenatide once weekly over 24 weeks in 252 patients with T2DM. The once-weekly formulation provided superior glycemic control compared with standard twice-daily dosing. The mean reduction in HbA1c was -1.6% in the exenatide once-weekly group and -0.9% in the exenatide twice-daily group (P < .0001).
The DURATION-6 trial compared once-weekly exenatide and daily liraglutide in 911 patients with T2DM. After 26 weeks, the mean reduction in HbA1c was 1.26% in the exenatide group and 1.48% in the liraglutide group (P < .05). Once-weekly exenatide was associated with fewer adverse gastrointestinal events than daily liraglutide, including less nausea (9.3% vs 20.4%), vomiting (3.7% vs 10.7%), and diarrhea (6.1% vs 13.1%). More than twice as many patients in the liraglutide group (5.3%) than in the exenatide group (2.6%) discontinued treatment due to adverse events. Patients in both groups had a modest reduction in body weight, with no significant differences between treatments.
Treatment Considerations
Nausea: Nausea and vomiting related to delayed gastric emptying are the most common adverse events associated with GLP-1 RAs. Nausea is mostly mild-to-moderate, and is most common at initiation of therapy; it tends to decrease with continuing treatment. In 1 prospective study, the frequency of nausea was similar during weeks 26 to 52 of treatment for patients treated with liraglutide 1.8 mg/d, liraglutide 1.2 mg/d, or sitagliptin 100 mg/d. In the LEAD-6 trial, the risk of nausea was significantly higher for patients treated with exenatide 10 µg twice daily compared with those treated with liraglutide 1.8 mg/d (P < .0001). In DURATION-6, nausea was less frequent among patients who received once-weekly exenatide (9.3%) than among those who received liraglutide (20.4%).
Weight loss: In a meta-analysis of GLP-1 RAs, the magnitude of weight loss was similar for exenatide once weekly (mean: -2.8 kg; 95% CI: -5.2 to -0.3 kg), exenatide twice daily (mean: -2.8 kg; 95% CI: -2.9 to -2.7 kg), and liraglutide (mean: -2.8 kg; 95% CI: -3.5 to -0.9 kg). Several studies have demonstrated that the weight loss associated with GLP-1 RA treatment is sustained for at least 2 years.
Use in patients on insulin therapy: Twice-daily exenatide has also been evaluated as add-on therapy in patients treated with glargine insulin. In the prospective trial, 259 patients who were taking glargine insulin were randomly assigned to add-on therapy with placebo or twice-daily exenatide. Compared with placebo, exenatide was associated with a greater reduction in HbA1c (-1.0% vs -1.7%), more favorable effects on body weight (+1.0 kg vs -1.8 kg), and a smaller increase in glargine dose (20 units vs 13 units). Patients had a similar risk of hypoglycemia regardless of treatment group. However, more patients in the exenatide group than in the placebo group dropped out of the study (13 vs 1).
Use in patients with comorbid renal impairment: Renal impairment appears to impact the clearance of exenatide in patients with T2DM, but does not affect the metabolism of liraglutide.This is because exenatide is eliminated predominantly via glomerular filtration, whereas no specific organ serves as the major route of elimination for liraglutide. Table 5 summarizes the recommended dosing for exenatide and liraglutide for patients with T2DM and comorbid renal impairment.
Table 5. GLP-1 Receptor Agonist Dosing in Patients with Renal Impairment
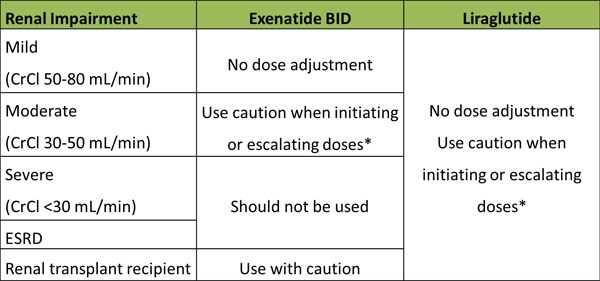
*Hypovolemia due to nausea/vomiting may worsen renal function
CrCl = creatinine clearance; ESRD = end-stage renal disease
Safety Considerations
Pancreatitis: In 2008, the Food and Drug Administration (FDA) issued an alert to healthcare professionals regarding the risk of pancreatitis with incretin-related drugs. The FDA report described several issues with incretin agents including:
- Reports of pancreatitis in liraglutide clinical trials.
- Postmarketing reports of pancreatitis in patients taking exenatide, including cases of hemorrhagic/necrotizing pancreatitis that resulted in patient deaths.
Later, an analysis from the FDA Adverse Event Reporting System (AERS) reported:
- A 6-fold increase of pancreatitis with sitagliptin or exenatide compared with other antidiabetes medications, including rosiglitazone, nateglinide, repaglinide, and glipizide.
- An increase in the reporting rate of pancreatic cancer in patients treated with sitagliptin or exenatide.
Despite the value of these safety warnings, the AERS has limitations for understanding true pancreatitis risk, including potential reporting bias. In addition, the AERS reporting system did not collect information on BMI, a known risk factor for pancreatitis.
Healthcare professionals should consider the following precautions related to pancreatitis when treating patients with incretin-based therapies.
- Adhere to label warnings
- Observe patients for symptoms of acute pancreatitis (persistent severe abdominal pain that may be accompanied by vomiting)
- Discontinue drug if pancreatitis is suspected
- Do not restart drug if pancreatitis is confirmed
- Consider other antihyperglycemic therapies in patients with a history of pancreatitis
Thyroid cancer: Liraglutide and once-weekly exenatide carry boxed warnings for thyroid C-cell tumors based on data from rodent models of T2DM. Clinical trials and postmarketing data show conflicting results regarding the effects of GLP-1 analogs on calcitonin levels, with small increases in calcitonin levels in some studies and reduced levels in others. To date, no definitive cases of medullary thyroid carcinoma related to liraglutide have been detected. No cases of thyroid cancer have been reported in the exenatide clinical trials.
Renal impairment: Postmarketing reports have also tracked renal outcomes in patients treated with GLP-1 RAs. To date, no evidence indicates that GLP-1 RAs are directly toxic to kidney cells. However, renal impairment may occur in patients who have experienced nausea, vomiting, diarrhea, and dehydration. In some cases, renal impairment has been observed in patients who are taking concurrent medication known to affect renal function or hydration status (eg, angiotensin-converting enzyme [ACE] inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], or diuretics). In addition, cases of patients requiring hemodialysis or transplantation have also been reported. Impaired renal function has also been observed in patients without known underlying renal disease.
In many cases, treatment-emergent renal impairment is reversible with supportive treatment and discontinuation of potentially causative agents. Current labeling includes a warning against GLP-1 RA use in patients with severe renal impairment or end-stage renal disease (ESRD). Furthermore, GLP-1 RAs should be used with caution in patients with a history of renal transplantation (exenatide and exenatide once weekly), and when initiating or escalating exenatide doses in patients with T2DM and comorbid renal impairment (exenatide, exenatide once weekly, and liraglutide).
No comments:
Post a Comment